- SITEMAP
- CONTACT US
- 8618267732328
News
Credibility ,the lifeblood of enterprise!
- Fittings
- Butt Welding Fittings
- Forged Fittings
- 180 Degree Elbows
- 90 Degree Elbows
- 60 Degree Elbows
- 45 Degree Elbows
- 30 Degree Elbows
- Equal Tee
- Reducing Tee
- Concentric Reducer
- Eccentric Reducer
- Lap Joint Stub End
- Outlets
- Cap
- Bend
- Cross
- Coupling
- Stainless Steel Lateral Tee
- Bellows Expansion Joints
- Flexible Metal Hose
- Non-Standard/Custom Fittings
- Bleed & Flushing Rings
- Types of Flanges
- Anchor Flanges
- Blind Flanges
- Expander Flanges
- High Hub Flanges
- Lap Joint Flanges
- Long Weld Neck Flanges
- Nipoflanges
- Orifice Flanges
- Plate Flanges
- Ring Type Joint Flanges
- Reducing Flanges
- Slip On Flanges
- Socket Weld Flanges
- Spectacle Blind Flanges
- Square Flanges
- Spades & Ring Spacers
- Threaded Flanges
- Welding Neck Flanges
Why stainless steel corrosion resistance?
Why stainless steel corrosion resistance?
Steel is made of iron and carbon, and stainless steel contains iron, carbon, and anywhere from 12-30% chromium. Stainless steel can contain other elements such as nickel and manganese, but chromium is the key element which makes it rust resistant. When the surface of normal steel is exposed to oxygen, it usually forms ferric oxide (Fe2O3) which has the well-known red rust color. Ferric oxide doesn’t form a continuous layer on the steel because the oxide molecule has a larger volume than the underlying iron atoms, and eventually spalls off leaving fresh steel exposed which then starts a deleterious rusting cycle. When stainless steel is exposed to oxygen, chromium oxide is created on the surface of the steel because chromium has a very strong affinity for oxygen. The chromium oxide is a very thin layer which doesn’t spall off, and it prevents further oxidation of the stainless steel. Even if stainless steel is scratched and the chromium oxide layer is removed, a new chromium oxide layer will form and protect the rest of the stainless steel beneath it. As long as there is sufficient chromium present, the chromium oxide layer will continue to protect the stainless steel and prevent it from rusting.
The passive layer forms because of the chromium added to stainless steel. Stainless steel must have at least 10.5% chromium in order for the passive layer to form. The more chromium that is added, the more stable the passive layer becomes, and the better the corrosion resistance. Other elements such as nickel, manganese, and molybdenum can be added to enhance stainless steel corrosion resistance.
Another requirement for the formation and maintenance of the passive layer is that the steel surface must be exposed to oxygen. Corrosion resistance is greatest when the steel is boldly exposed and the surface is maintained free of deposits. If passivity is destroyed under conditions that do not permit restoration of the passive film, then stainless steel will corrode much like a carbon or low-alloy steel. For example, covering a portion of the surface – for example, by biofouling, painting, or installing a gasket – produces an oxygen-depleted region under the covered region. The oxygen-depleted region is anodic relative to the well-aerated boldly exposed surface, possibly resulting in the corrosion of the covered region.
Stainless steel composition is stable
The actual use of stainless steel environment is very complex, ordinary chromium oxide passive film can not fully adapt to a variety of environments. According to the actual situation in the stainless steel by adding molybdenum, copper, nitrogen and other elements, in order to further improve the corrosion resistance of the passive film.
Adding molybdenum, when the corrosive medium near the substrate will promote the passivation of the stainless steel substrate to form a passive film corrosion; add copper to the surface of stainless steel passive film contains CuCl, and corrosive media does not play a role in improving corrosion resistance; Addition of nitrogen makes the passivation film rich in CrN, improve the chromium concentration in the passive film, and enhance the corrosion resistance of stainless steel.

Pitting in 304 stainless steel
Under certain circumstances, the passive layer can break down at localized spots on a well exposed stainless steel surface. When this happens, the metal can corrode in the localized spots. This is called pitting corrosion. One common cause of pitting corrosion is exposure to aqueous environments that contain chloride. Examples are coastal atmospheres, road salt combined with rain water, and even tap water containing high levels of chloride.
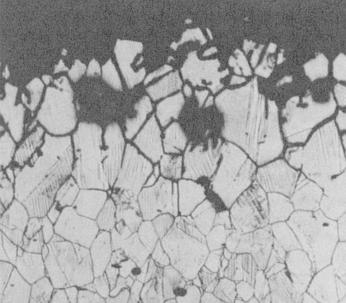
Intergranular corrosion of 304 stainless steel
During the fabrication of stainless steel components or structures it is possible to degrade the corrosion resistance. This occurs when austenitic stainless steels (e.g. 304) are exposed to temperatures between about 425 °C (797 °F) and 870 °C (1598 °F). If the exposure time is too long, then the areas near the metal’s grain boundaries lose their corrosion resistance and can be preferentially attacked when exposed to a corrosive environment. The grains fall out and the metal loses strength. The increased susceptibility to corrosion by this change in microstructure is called sensitization.

Tel No:+86-18267732328 / Email:[email protected]
Address:Longwan District, Wenzhou, Zhejiang Province, China.
Copyright Notice © www.yaang.com Yaang Pipe Industry Co., Limited All rights reserved.
Yaang Pipe Industry Co., Ltd. is an international supplier of piping solutions for flange, butt welding fittings, socket welding fittings and threaded fittings. Our products are widely used in different industrial fields, including oil and gas, chemical industry, petrochemical industry, power plant, pulp and paper industry, environmental and water conservancy engineering, engineering projects, etc.





